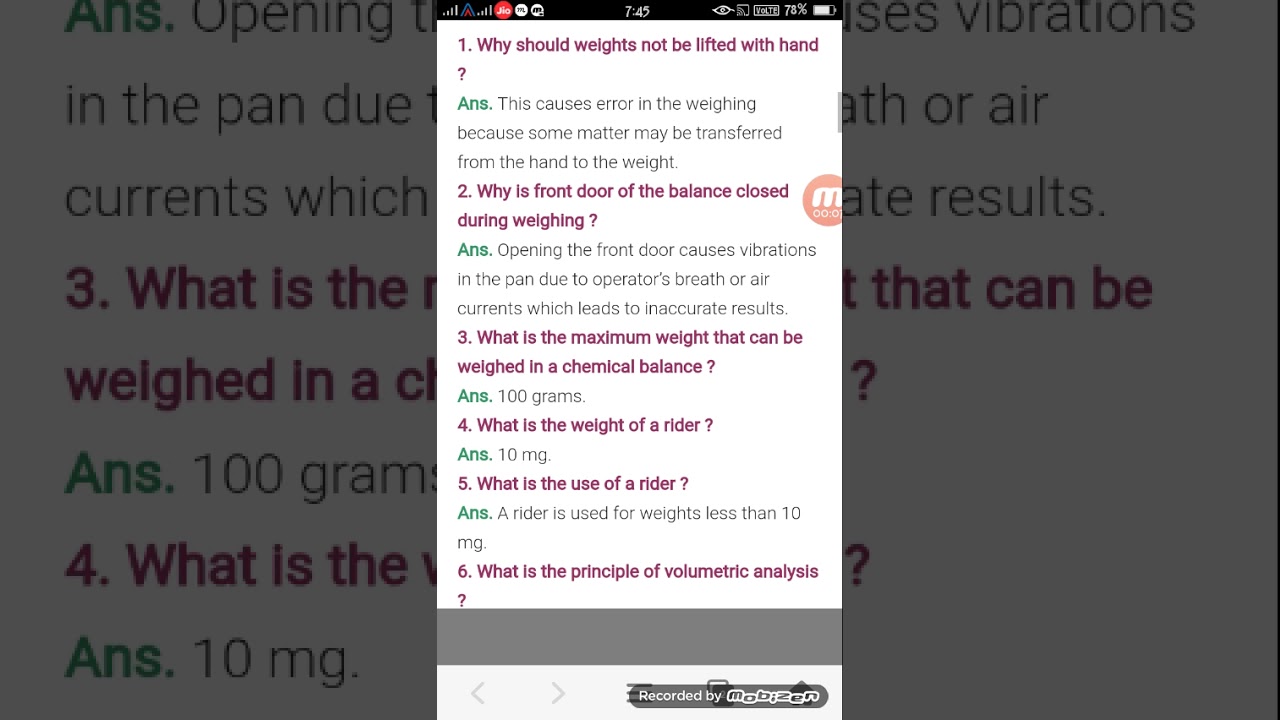
Chemistry Titration Practical Viva Questions . In close proximity to the endpoint, the action of the. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. Why is diluted sulphuric acid [h 2 so 4] added while preparing. The process of adding one solution from the burette to another in the titration flask in order to complete the chemical reaction involved, is known as titration. the document contains 20 questions and their answers related to titration experiments in chemistry practicals.
from www.youtube.com
the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In close proximity to the endpoint, the action of the. Why is diluted sulphuric acid [h 2 so 4] added while preparing. The process of adding one solution from the burette to another in the titration flask in order to complete the chemical reaction involved, is known as titration. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. the document contains 20 questions and their answers related to titration experiments in chemistry practicals.
Class 12 titration chemistry practical viva question with answers YouTube
Chemistry Titration Practical Viva Questions chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. Why is diluted sulphuric acid [h 2 so 4] added while preparing. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the document contains 20 questions and their answers related to titration experiments in chemistry practicals. The process of adding one solution from the burette to another in the titration flask in order to complete the chemical reaction involved, is known as titration. In close proximity to the endpoint, the action of the.
From www.youtube.com
Important Viva Questions for Chemistry Practical CBSE Class 12 Chemistry Titration Practical Viva Questions the document contains 20 questions and their answers related to titration experiments in chemistry practicals. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. Why is diluted sulphuric acid [h 2 so 4] added while preparing. the titration of potassium permanganate (kmno 4) against mohr salt is an example. Chemistry Titration Practical Viva Questions.
From issuu.com
CHEMISTRY LAB VIVA Questions by ramesh kumar issuu Chemistry Titration Practical Viva Questions the document contains 20 questions and their answers related to titration experiments in chemistry practicals. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In close proximity to the endpoint, the action of the. The process of adding one solution from the burette to another in the titration flask in order. Chemistry Titration Practical Viva Questions.
From www.scribd.com
Class 11th Viva Questions Chemistry PDF Titration Chemistry Chemistry Titration Practical Viva Questions chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the document contains 20 questions and their answers related to titration experiments in chemistry practicals. Why is diluted sulphuric acid [h 2 so 4]. Chemistry Titration Practical Viva Questions.
From www.thinkswap.com
Stage 2 Chemistry Titration Practical Chemistry Year 12 SACE Chemistry Titration Practical Viva Questions the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The process of adding one solution from the burette to another in the titration flask in order to complete the chemical reaction involved, is known as titration. the document contains 20 questions and their answers related to titration experiments in chemistry practicals.. Chemistry Titration Practical Viva Questions.
From www.savemyexams.co.uk
Titrations (1.7.2) Edexcel A Level Chemistry Revision Notes 2017 Chemistry Titration Practical Viva Questions the document contains 20 questions and their answers related to titration experiments in chemistry practicals. Why is diluted sulphuric acid [h 2 so 4] added while preparing. The process of adding one solution from the burette to another in the titration flask in order to complete the chemical reaction involved, is known as titration. the titration of potassium. Chemistry Titration Practical Viva Questions.
From learningintonaco7o.z13.web.core.windows.net
Chemistry Titration Questions And Answers Chemistry Titration Practical Viva Questions chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. the document contains 20 questions and their answers related to titration experiments in chemistry practicals. The process of adding one solution from the burette to another in the titration flask in order to complete the chemical reaction involved, is known as. Chemistry Titration Practical Viva Questions.
From www.studocu.com
Experiment 7 Titrations Experiment 7 Titrations Required reading Chemistry Titration Practical Viva Questions Why is diluted sulphuric acid [h 2 so 4] added while preparing. In close proximity to the endpoint, the action of the. The process of adding one solution from the burette to another in the titration flask in order to complete the chemical reaction involved, is known as titration. the document contains 20 questions and their answers related to. Chemistry Titration Practical Viva Questions.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Chemistry Titration Practical Viva Questions Why is diluted sulphuric acid [h 2 so 4] added while preparing. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the document contains 20 questions and their answers related to titration experiments in chemistry practicals. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against. Chemistry Titration Practical Viva Questions.
From www.scribd.com
Chemistry Viva Question PDF Titration Chemistry Chemistry Titration Practical Viva Questions Why is diluted sulphuric acid [h 2 so 4] added while preparing. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. The process of adding one solution from the burette to another in the titration flask in order to complete the chemical reaction involved, is known as titration. the titration. Chemistry Titration Practical Viva Questions.
From www.scribd.com
Chemistry Viva Questions Titration Redox Chemistry Titration Practical Viva Questions In close proximity to the endpoint, the action of the. Why is diluted sulphuric acid [h 2 so 4] added while preparing. the document contains 20 questions and their answers related to titration experiments in chemistry practicals. The process of adding one solution from the burette to another in the titration flask in order to complete the chemical reaction. Chemistry Titration Practical Viva Questions.
From www.scribd.com
VIVA Question Chem Prac PDF Titration Chemistry Chemistry Titration Practical Viva Questions Why is diluted sulphuric acid [h 2 so 4] added while preparing. In close proximity to the endpoint, the action of the. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. the document contains 20 questions and their answers related to titration experiments in chemistry practicals. the titration of. Chemistry Titration Practical Viva Questions.
From www.vrogue.co
Gcse Chemistry Aqa 9 1 How To Do A Titration Teaching vrogue.co Chemistry Titration Practical Viva Questions Why is diluted sulphuric acid [h 2 so 4] added while preparing. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. In close proximity to the endpoint, the action of the. The process of adding one solution from the burette to another in the titration flask in order to complete the. Chemistry Titration Practical Viva Questions.
From dxodobzvw.blob.core.windows.net
Titration Lab Follow Up Questions at Wen blog Chemistry Titration Practical Viva Questions the document contains 20 questions and their answers related to titration experiments in chemistry practicals. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. The process of adding one solution from the burette. Chemistry Titration Practical Viva Questions.
From www.scribd.com
Chemistry Practical VIVA Question XII PDF Titration Chemistry Chemistry Titration Practical Viva Questions The process of adding one solution from the burette to another in the titration flask in order to complete the chemical reaction involved, is known as titration. Why is diluted sulphuric acid [h 2 so 4] added while preparing. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. the document. Chemistry Titration Practical Viva Questions.
From www.youtube.com
Redox Titrations A level Chemistry Question Walkthrough YouTube Chemistry Titration Practical Viva Questions chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the document contains 20 questions and their answers related to titration experiments in chemistry practicals. The process of adding one solution from the burette. Chemistry Titration Practical Viva Questions.
From www.youtube.com
Class 12 titration chemistry practical viva question with answers YouTube Chemistry Titration Practical Viva Questions the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. Why is diluted sulphuric acid [h 2 so 4] added while preparing. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. The process of adding one solution from the burette to another in the. Chemistry Titration Practical Viva Questions.
From www.scribd.com
Practicals Viva PDF Titration Chemistry Chemistry Titration Practical Viva Questions the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In close proximity to the endpoint, the action of the. the document contains 20 questions and their answers related to titration experiments in chemistry practicals. Why is diluted sulphuric acid [h 2 so 4] added while preparing. The process of adding one. Chemistry Titration Practical Viva Questions.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Chemistry Titration Practical Viva Questions the document contains 20 questions and their answers related to titration experiments in chemistry practicals. Why is diluted sulphuric acid [h 2 so 4] added while preparing. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. the titration of potassium permanganate (kmno 4) against mohr salt is an example. Chemistry Titration Practical Viva Questions.